<< 1 >>
Rating: 
Summary: Handbook of Computer & Computerized System Validation
Review: As consultants to the Pharmaceutical Industry in the area of validation we found Handbook of Computer and Computerized System Validation for the Pharmaceutical Industry (1Stbooks Library (Series).) by Stephen Robert Goldman to be an excellent primer on how to validate computers and computer systems. The book presents a practical approach, easy to follow, and is up to date with current trends. The author clearly knows his subject.
Rating: 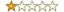
Summary: Disappointed
Review: Horrible repetitive heading style and sleep-inducing writing style led to quick frustration. The author makes computer validation as exciting as a baloney sandwich on white bread.
I gave up trying to read this book and hope to find one that presents the material in a better fashion
Rating: 
Summary: clearly understood
Review: I am not new to validation having worked in a highly regulated industry for several years. And just reciently, I found the need to upgrade current systems in my engineering business. I used this handbook, and I am happy to say that Mr. Goldman covers the subject very well and is so complete that after reading it, anyone can create a workable system without using another source. The book is clearly written and is easy to understand.
Rating: 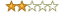
Summary: Shockingly disappointed....
Review: I am not usually one to 'bash' a book, but I couldn't resist commenting here. I will give Mr. Goldman the stars for an incredible outline/agenda. He really does give to thoroughness as for what topics to address during the validation process, which is more than most do. BUT where this book REALLY falls short is in supporting details on those topics. The heading fonts are crazy and VERY difficult to follow. He repeats the same outline for each of the validation phases, and repeats the content from one phase to the next... just replacing the title of each section. Example, under Hardware Design Specifications, there is a section for Warnings/Notes/General Info.."Warnings, notes, general or safety information applicable to the Hardware Design Specification SOP are listed in this section." When you move ahead a few sections to Unit Testing, you get, for Warnings/Notes/General Info ..."Warnings, notes, general or safety information applicable to the Unit Testing Protocol are listed in this section.
SO, so many pages of the 450 page book are repeated. By reorganizing the book, he could have cut 300 pages.
I would have hoped to find supporting examples of Warnings, Notes or General Information that we as document writers might want to consider. I know that each of our situations is different and our environments are different, but give us food for thought. Things to consider. Make me say, "Oh, I didn't think of that." For those poor souls that have been thrusted into writing documents for validated systems and are wandering blindly, this book will give you a full V-model of documents that your system will require, but it will fall WAY short of letting you know what each of those documents should contain and shouldn't contain. Please don't blindly think that because you've created V-model documents that the auditors will nod and smile at you. You will require some due-diligence beyond this book. Good luck! -Pharmaceutical Technical Writer.
Rating: 
Summary: Valuable reference
Review: Mr. Goldman's book is a valuable reference tool for the experienced as well as novice validation specialist. The subject matter is complete and provides a high-level overview of the requirements for a successful and compliant validation. I highly recommend this book for those in the validation business.
Rating: 
Summary: Indispensable reference
Review: The editorial review gave the impression that this document would give examples of the SOPs and documents required to create software quality assurance procedures for a pharma company. The book is largely repetitive chapters for each category of SOP and document, with fine detail on easy things like the contents of a title page, and vague handwaving for the important things, such as "The SOP should contain instrucitons and procedures that enable the creation of a XXX and the performance of XXX in accordance with its specifications. The XXX is prepared as a stand-alone document for all projects. It is not good practice to include it as part of other documents in an attempt to reduce the amount of workload or paperwork." and then goes on to recommend the GAMP guide as a source for the procedures. Needless to say, this does not make for a valuable book. So far, I'm finding the greatest value in the two-page appendix with its grid of SOPs and responsibilities, but even that is bloated with useless columns.
Rating: 
Summary: very informative
Review: This is a great reference book for people in the pharmaceutical industry who need to know the rules for properly replacing a computer system and documenting the procedures that need to be followed in order to meet due diligence and compliance regulations. Each chapter addresses a different aspect of the process.
<< 1 >>
|